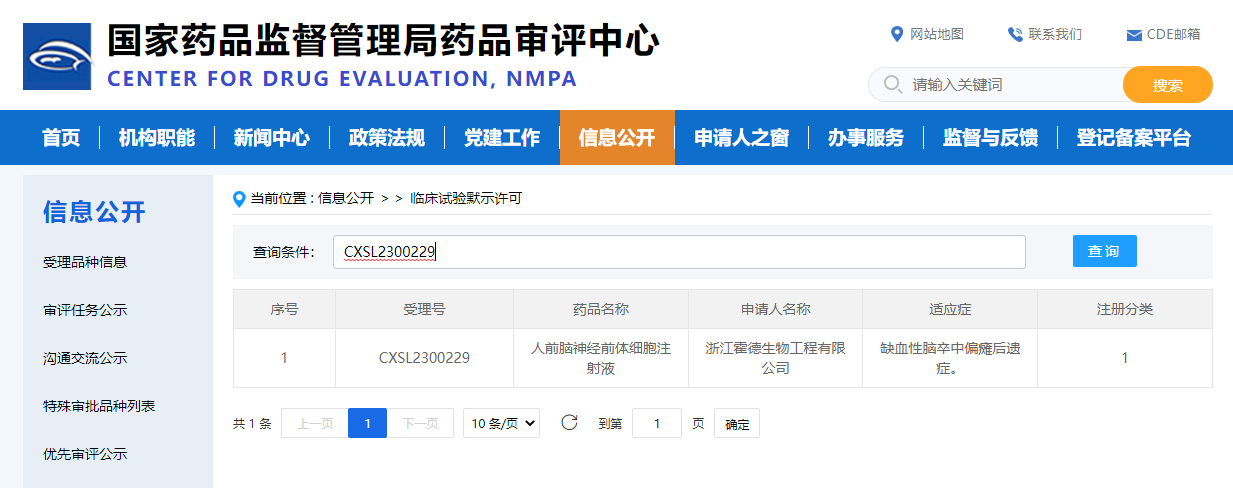
Hopstem is proud to announce that its iPSC-derived cell therapy product, hNPC01 injection, aimed at treating the residual effects of ischemic stroke, has received clinical trial implication approval from the China National Medical Products Administration's Center for Drug Evaluation (CDE) on June 21, 2023. (Acceptance number: CXSL2300229)
The hNPC01 cell injection is the world's first IND-approved pluripotent stem cell-derived forebrain neural progenitor cell product and also the first neuro iPSC cell product in China to have received CDE clinical trial implication approval. Hopstem's founder and CEO, Dr. Jing Fan, commented, "It was a great challenge to develop a universal neural cell therapy product derived from iPSCs for intracranial administration, and we are delighted that our first iPSC-derived cell product, hNPC01, has received clinical trial implication approval from CDE. We look forward to fully exploring the safety and efficacy of this product in the upcoming clinical research. We thank our team for their years of hard work, as well as our shareholders, patients, and industry partners for their long-term support. Hopstem will continue to uphold the values of “patient first” and “consistent accumulation to make a success” and develop more high-quality cell therapy products, conduct rigorous and compliant clinical research and trials to verify the safety and efficacy of our products, and ultimately benefit more patients as soon as possible."
This hNPC01 injection, which has received CDE clinical trial implication approval, is the first domestically developed iPSC-derived forebrain neural progenitor cell product in China and will be the first of its kind to undergo IND filing in the United States. Currently, the only similar product in the international market is the iPSC-derived forebrain neural progenitor cell therapy for residual effects of cerebral infarction led by Dr. Gary Steinberg at Stanford University. This project received funding from the National Institutes of Health (NIH) and Stanford University and started clinical research in April 2022.
FDA Granted Hopstem’s hNPC01 for Chronic Ischemic Stroke Approval to Enter Phase 2/3 Adaptive Pivotal Clinical Trial with Bridging Study

Hopstem Announces FDA Granted iPSC Therapy hNPC01 Injection Fast Track Designation, Accelerating Global Breakthrough in Treatment of Cerebral Neural Injury Diseases

Hopstem Won First Place at 2024 SAPA Investment Forum & online Road Show with Innovative iPSC Cell Therapy for Chronic Stroke

Hopstem Biotechnology at 2024 CASSS CGPT Symposium
